Authors: Shera Wanner MD & John Walsh MD*
Background
Pneumonia is generally defined as inflammation of the lung parenchyma, typically due to an infectious etiology–bacterial, viral, or fungal. Grossly, pneumonia can be divided into
- Lobar pneumonia: involvement of the entire lung lobe/segment, often caused by Streptococcus pneumoniae, presents with sudden onset and uniform consolidation on imaging.
- Bronchopneumonia (lobular): patchy inflammation centered along airways (bronchi and bronchioles) with intervening islands of normal lung parenchyma, respectively. It is caused by a variety of organisms and presents with a more insidious onset with patchy consolidation on imaging.
 Image: diagram of lobar vs. bronchopneumonia. (Image credit: Happiest Health).
Image: diagram of lobar vs. bronchopneumonia. (Image credit: Happiest Health).
- The pathogenesis of pneumonia is the result of one of four mechanisms: inhalation, aspiration, hematogenous spread, or direct extension from infectious foci. Bronchopneumonia is often the result of pathogenic inhalation or aspiration.
- Evaluation and treatment of pneumonia is dependent upon known and/or suspected etiologic agents and environments–considerations which can be useful context in the autopsy setting as well.
| Community-Acquired Pneumonia (CAP) | Pneumonia acquired outside of the healthcare setting.
|
| Hospital-Acquired Pneumonia (HAP) | Acquired >48 hrs after admission.
|
| Healthcare-Associated Pneumonia | Acquired in a non-hospital healthcare setting such as nursing home or dialysis center. |
Quick Tips at Time of Autopsy
Clinical History
The best autopsy results can be obtained when the autopsying pathologist pre-plans and thinks through their differential diagnosis before beginning. This pre-planning should include what testing/sampling is needed to evaluate each potential diagnosis. In the case of decedent’s whom an infectious cause of death is being considered this may include forethought into the decedent’s demographics (elderly, infant, immunocompromised), types of infectious agent (viral, bacterial, fungal), need for additional PPE or neg pressure autopsy suite, types of cultures desired (blood, CSF, urine, tissue [securing wet unfixed tissue for culture or molecular], tissue swab, viral nasopharyngeal, or other – see the The Post-mortem Cultures article for more information), coordination with the microbiology lab, the procurement and organization of appropriate sampling tools in the autopsy suite (sterile scalpel blades, sterile swabs, blood culture bottles, etc), ensuring the correct orders are placed in the medical record, and a strategy for histology sampling. While this is true generally for all autopsies, it is mentioned here because patients with a suspected infectious cause of death require slightly more thought up front, so that opportunities such as sterile cultures are not missed. (Again, see The Post-mortem Cultures article for additional information).
- Pneumonia is the third most commonly missed antemortem diagnosis confirmed at autopsy. Therefore, its presence may not be suspected prior to death (Roulson 2005).
- Pulmonary emboli, ischaemic heart disease/myocardial infarction and pneumonia are often confused with each other in the clinical setting, so an elevated index of suspicion for pneumonia in cases with concern for pulmonary embolism and/or myocardial infarction is warranted (Roulson 2005).
- Patients often present with fever, purulent sputum, shortness of breath, and declining oxygenation status.
- Medical records may contain pre-mortem diagnostic information such as:
- Sputum and blood culture results
- Elevated WBC with left shift
- Thrombocytopenia, low fibrin (DIC)
- Hx of preceding viral infection
- Radiographic findings (see images below)
- Risk factors include additional comorbidities and/or immunocompromised (including infants and the elderly).

Image: Lobar pneumonia demonstrating opacities diffusely within a single lobe on x-ray. (Image credit: Radiology Master Class).
 Image: Bronchopneumonia demonstrating multifocal opacities centered on airways. (Image credit: Mohammad Osama Yussein Yonso on Radiopedia.)
Image: Bronchopneumonia demonstrating multifocal opacities centered on airways. (Image credit: Mohammad Osama Yussein Yonso on Radiopedia.)
Ancillary testing
- Tissue culture (lung or spleen) is recommended in some situations. See the Postmortem Cultures article for additional information.
- Cold agglutinins: Not specific for Mycoplasma but if high titer (>1:64) cold agglutinin in community acquired pneumonia, greater likelihood of Mycoplasma infection. Tip: Can compare with premortem clinical findings of atypical pneumonia (e.g., prolonged constitutional symptoms, nonproductive cough, headache, sore throat, and low-grade fevers).
- Can consider serologic testing for Mycoplasma, Chlamydia, Influenza A/B, adenovirus, and RSV antibodies or rapid PCR organism isolation.
- Legionella urine antigen test.
External examination
- Generally consists of documenting evidence of treatment:
- Endotracheal tube if underlying pneumonia contributed to acute hypoxemic respiratory failure
- Chest tube in the setting of empyema requiring drainage
- Central and/or peripheral intravenous lines for fluid resuscitation and IV antibiotics
- Additionally, signs of sepsis may be present (e.g. petechiae in DIC, jaundice, pallor).
Internal Examination
Lungs/ chest cavity
- Identifying areas concerning for pneumonia grossly is not always reliable. In studies, only half of cases thought to have pneumonia by gross exam are confirmed on histology (42.9% PPV in Hjorth 1995, 59% PPV in Bernardi 2005).
- Additionally, cases of pneumonia are often not detected on gross examination but are detected histologically with appropriate sampling (approximately 1/3rd of cases of pneumonia are not suspected grossly; Hjorth 1995 and Bernardi 2005).
- Adequate sampling includes representative tissue from all lobes (ideally BOTH peripherally and centrally samples – a minimum 10 sections total).
- Fibrinous adhesions may be present in the setting of prolonged/chronic infection and/or infection associated with empyema.
 Image: The right lung is diffusely discolored from overt purulence. The right lung makes for a good comparison to an edematous but otherwise not discolored lung. (Image: Meagan Chambers/University of Washington).
Image: The right lung is diffusely discolored from overt purulence. The right lung makes for a good comparison to an edematous but otherwise not discolored lung. (Image: Meagan Chambers/University of Washington).
- A slimy mucoid appearance is characteristic for Klebsiella spp.
- The presence of cavitary lesions common in S. aureus and P. aeruginosa.
- Lobar pneumonia (often S. pneumoniae and K. pneumoniae) is the result of progression through 4 inflammatory response stages which can be observed grossly:
- Congestion: heavy, red lung occurring within the first 24 hours
- Red hepatization: red blood cells, neutrophils, and fibrin infiltrate the lungs’ alveolar fluid (consolidation). This causes the lungs to appear red and firm (similar to liver). Red hepatization usually begins 2–3 days after consolidation and lasts for 2–4 days.
- Gray hepatization: red blood cells begin to break up, causing the lungs to become grayish-brown or yellow in color. The lungs will become drier, too, further taking on a liver-like consistency. This usually occurs 4-6 days into the cycle.
- Resolution: The lung may return to baseline as the consolidated exudate is digested.
 Image: Congested lungs (red and edematous) are not uncommon at autopsy and can signal a variety of underlying conditions including, but not limited to pneumonia. In this image there is also a thrombus in one of the vessels (arrow). (Image: Meagan Chambers/University of Washington).
Image: Congested lungs (red and edematous) are not uncommon at autopsy and can signal a variety of underlying conditions including, but not limited to pneumonia. In this image there is also a thrombus in one of the vessels (arrow). (Image: Meagan Chambers/University of Washington).
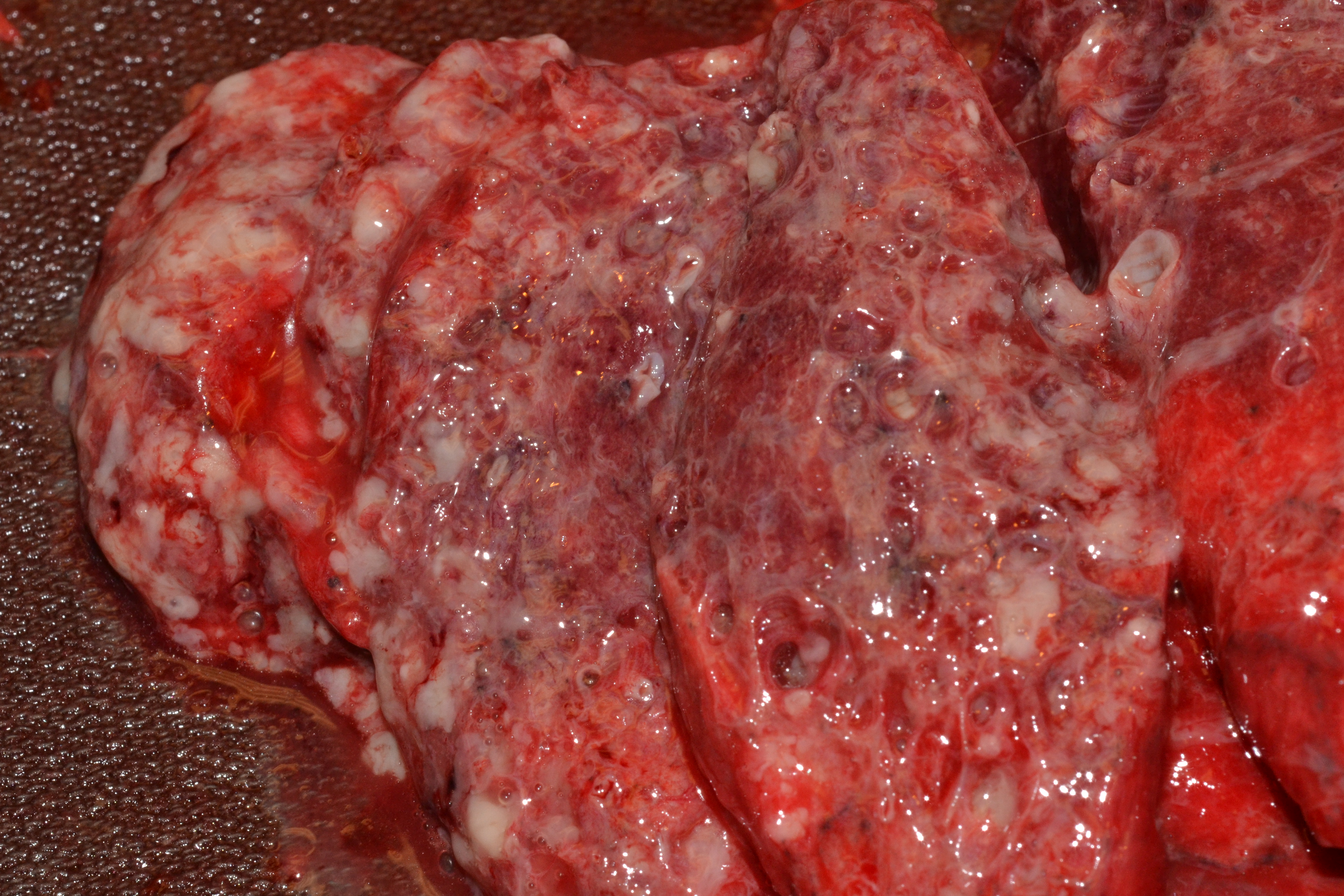
 Image: Red hepatization is present in the upper left half of this image, while congested parenchyma is present in the bottom right of the lung. Multiple circumscribed abscesses are also present. Click here for an annotated version of this image. (Image credit: Meagan Chambers/University of Washington).
Image: Red hepatization is present in the upper left half of this image, while congested parenchyma is present in the bottom right of the lung. Multiple circumscribed abscesses are also present. Click here for an annotated version of this image. (Image credit: Meagan Chambers/University of Washington).
 Image: The lower lobe of this lung is diffusely consolidated with tan-white material infiltrates consistent with gray hepatization. (Image credit: Meagan Chambers/University of Washington).
Image: The lower lobe of this lung is diffusely consolidated with tan-white material infiltrates consistent with gray hepatization. (Image credit: Meagan Chambers/University of Washington).
- Bronchopneumonia generally affects the lungs bilaterally, though type and extent of parenchymal involvement can be highly variable requiring histological confirmation.
- Basilar regions often contain most conspicuous areas of change.
- Consolidation (filling of airspaces with exudate) is poorly circumscribed, and yellow to gray-red in color.
- May be limited to one lobe or involve multiple lobes.
- Bronchopneumonia may be difficult to visualize grossly.
Importantly, small foci of consolidation can coalesce and resemble lobar pneumonia.
- Gross descriptions should include distribution of consolidation, presence of necrosis and/or abscess (if any).
- Ensure that histology sections are representative of both affected and unaffected lung parenchyma and small airways. Dedicated sections of the interface between normal and abnormal are more useful than solid lesion areas.
 Image: (left) Early consolidations can be seen in the lower right half of the image as dark red parenchyma with and area of scattered tan-yellow deposits consistent with neutrophilic infiltration (between the yellow arrows). This can be hard to distinguish from bronchopneumonia (right) on gross examination alone. (Image credit: (left) Meagan Chambers/University of Washington, (right) The Japanese Society of Pathology).
Image: (left) Early consolidations can be seen in the lower right half of the image as dark red parenchyma with and area of scattered tan-yellow deposits consistent with neutrophilic infiltration (between the yellow arrows). This can be hard to distinguish from bronchopneumonia (right) on gross examination alone. (Image credit: (left) Meagan Chambers/University of Washington, (right) The Japanese Society of Pathology).
- Take into consideration mimics of gross consolidation such as pulmonary hemorrhage, interstitial pneumonia, and lung adenocarcinoma.
Quick Tips at Time of Histology Evaluation
- Intra-alveolar fibrinopurulent exudate with neutrophils are essential features of pneumonia.
Lobar pneumonia
- Uniform inflammatory infiltrate, the changes are at the same stage throughout the entire lobe
- Early stage: vascular engorgement, intra-alveolar fluid with few neutrophils and often bacterial colonies
- Red hepatization: confluent exudate with intra-alveolar neutrophils, red cells and fibrin
- Gray hepatization: disintegration of red cells and the persistence of a fibrinosuppurative exudate
- Resolution: As the inflammatory reaction resolves, the consolidated exudate is digested, leaving a granular semi-solid fluid to be resorbed. Alternatively, the intra-alveolar exudate can undergo organization, in which alveoli are filled by nodules of fibroblasts, collagen, and macrophages.

Image: Early evolving aspiration pneumonia in the congested phase; diffuse edema, occasional neutrophils, and foreign body material (circle) are seen. (Image credit: Meagan Chambers/University of Washington).
 Image: Confluent neutrophils within airspaces with or without associated hemorrhage is characteristic of the red hepatization phase in lobar pneumonia. (Image credit: Meagan Chambers/University of Washington).
Image: Confluent neutrophils within airspaces with or without associated hemorrhage is characteristic of the red hepatization phase in lobar pneumonia. (Image credit: Meagan Chambers/University of Washington).
 Image: The gray hepatization phase contains neutrophils, clumps of fibrin, and fragmented red blood cells. (Image credit: Meagan Chambers/University of Washington).
Image: The gray hepatization phase contains neutrophils, clumps of fibrin, and fragmented red blood cells. (Image credit: Meagan Chambers/University of Washington).
- Of note, histologic findings of pneumonia may overlap with findings of diffuse alveolar damage including hyaline membranes in the exudative stage and interstitial inflammation/fibrosis in the exudative phase. (See the histology section of the COVID-19 article for more information). Of note, DAD with prominent organizing pneumonia is also called organizing DAD.
Bronchopneumonia
- Different stages of inflammation in various areas, in contrast to lobar pneumonia
- Patchy, intra-alveolar fibrinopurulent exudate with neutrophils
 Image: This low-power image shows the spatial heterogeneity of bronchopneumonia. The dark purple areas are microabscesses; these have areas of relatively normal lung parenchyma between them, in contrast to lobar pneumonia. (Image credit: Meagan Chambers/University of Washington).
Image: This low-power image shows the spatial heterogeneity of bronchopneumonia. The dark purple areas are microabscesses; these have areas of relatively normal lung parenchyma between them, in contrast to lobar pneumonia. (Image credit: Meagan Chambers/University of Washington).
Aspiration pneumonia
- Histologically identified by food particles with an associated inflammatory component. The inflammation can include multinucleated giant cells, granulomatous inflammation, and/or abscesses.
- The presence of upper airway epithelium in alveoli is also evidence of aspiration.
- Food particles can also end up in terminal airways in the perimortem period, as with aspiration during CPR. This will not have an associated inflammatory infiltrate and should be considered an incidental finding as opposed to contributing to the cause of death.
 Image: Composite of aspirated oral contents. The top left demonstrates meat (skeletal muscle) in a large airway. Top right is vegetable matter in a large airway (note the thick box-like cell walls of the plant matter). Bottom left is additional vegetable matter with an associated giant cell reaction denoting true ante-mortem aspiration. The bottom right is a small cluster of bacteria (red arrow) and respiratory epithelium (including cilia – black arrow) without an associated inflammatory reaction – most consistent with perimortem aspiration such as from CPR. (Image credits: Meagan Chambers/Stanford University).
Image: Composite of aspirated oral contents. The top left demonstrates meat (skeletal muscle) in a large airway. Top right is vegetable matter in a large airway (note the thick box-like cell walls of the plant matter). Bottom left is additional vegetable matter with an associated giant cell reaction denoting true ante-mortem aspiration. The bottom right is a small cluster of bacteria (red arrow) and respiratory epithelium (including cilia – black arrow) without an associated inflammatory reaction – most consistent with perimortem aspiration such as from CPR. (Image credits: Meagan Chambers/Stanford University).
Immunohistochemistry
| Gram stain | Gram+ bacteria = blue
Gram- bacteria = red |
| GMS | Primarily for fungal detection, bacteria may occasionally stain positive. *Do not confuse organisms with yeast* |
| PAS | Positive in fungal infections |
| Mucicarmine | Cryptococcus neoformans |
Quick Tips for Autopsy Report
- Gross findings may not always correlate with what is seen on histology; histology is the gold standard for the final diagnosis.
- Tissue culture can be immensely helpful in patient scenarios where an unknown pathogen was treated or a suspected pathogen was unresponsive to therapy, however, these culture findings must be interpreted cautiously as tissue contamination at the time of autopsy is difficult to mitigate. (See the Postmortem Cultures Article for more information on interpreting these tests in the autopsy setting).
- When crafting COD statements, take into consideration the circumstances under which the infection was developed (e.g., community- or hospital-acquired), underlying risk factors, as well as infection time course and response to therapy (extrapolated from gross and microscopic findings).
- As per CDC guidelines for death certification, include the organism name in the cause of death when possible
- e.g. “Community Acquired pan lobar pneumonia due to Streptococcus pneumoniae infection.”
Recommended References
- Barson, William J. “Epidemiology, pathogenesis, and microbiology of community-acquired pneumonia in adults.” In: UpToDate. (Accessed May 2024).
- Litzky, Leslie A. “Bronchopneumonia.” In: Expertpath. (Accessed May 2024).
Additional Citations
- Bernardi FD, Saldiva PH, Mauad T. Histological examination has a major impact on macroscopic necropsy diagnoses. J Clin Pathol. 2005 Dec;58(12):1261-4. doi: 10.1136/jcp.2005.027953. PMID: 16311344; PMCID: PMC1770793.
- Hjorth L, Jensen HS, Noer H. Akutte lungeinfektioner ved obduktion. Undersøgelse af klinisk og makroskopisk diagnostik ved obduktion sammenholdt med mikroskopiske obduktionsfund [Acute pulmonary infections at autopsy. A study of clinical and macroscopic diagnoses at autopsy compared with microscopic autopsy findings]. Ugeskr Laeger. 1995 Dec 4;157(49):6873-6. Danish. PMID: 7491732.
- Hunt CR, Benbow EW, Knox WF, McMahon RFT, McWilliam LJ. Can histopathologists diagnose bronchopneumonia? J. Clin. Pathol. 1995; 48; 120–123.
- Nseir, Saad & Marquette, Charles-Hugo. “Diagnosis of hospital-acquired pneumonia: post-mortem studies.” Infectious Disease Clinics of North America. 2003, Dec.; 17(4): 707-716. doi: https://doi.org/10.1016/S0891-5520(03)00075-8
- Roulson J, Benbow EW, Hasleton PS. “Discrepancies between clinical and autopsy diagnosis and the value of post mortem histology; a meta-analysis and review.” ”Histopathology. 2005 Dec.; 47(6): 551-9. doi:10.1111/j.1365-2559.2005.02243.x. PMID: 16324191.
- Turner, Gareth D.H., Bunthi, Charatdao, Wonodi, Chizoba B., et. al. The role of postmortem studies in pneumonia etiology research. Clinical Infectious Diseases. 2012, Apr.; 54(2): 165-171. doi:10.1093/cid/cir1062
*The views expressed in this article are those of the author and do not necessarily reflect the official policy or position of the Department of Navy, Armed Forces Medical Examiner System, Uniformed Services University of Health Science, DHA, Department of Defense, or the US Government. The authors report no conflict of interest or sources of funding.